Twinning
| Two quartz crystals that have intergrown in the same space at the same time are called twinned crystals. The relationship between these two crystals is defined by a Twin Law. |
Japan Twin Law |
|
This presentation follows the criteria set by Clifford Frondel in Dana's "The System of Mineralogy", Seventh Edition, Volume III "Silica Minerals". All the twinning examples illustrated here are based only on the visual appearance of the crystals. Should anyone want to (a) dispute any of this information, (b) provide a more detailed explanation of these phenomenon, or (c) provide better examples please contact me at rjyd@cox.net. |
|
|
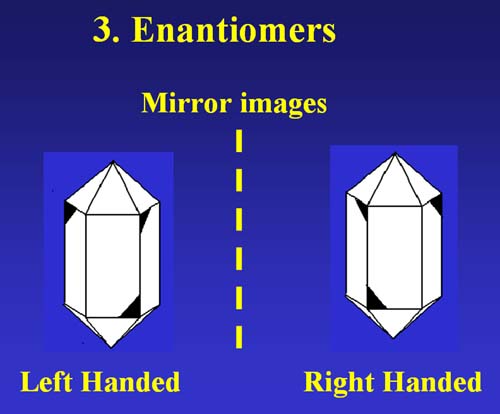
Twinning is not unique to quartz crystals nor minerals in general. There are two basic types of twinning in quartz crystals. There are Parallel Axis Twins that include the Dauphine Law, the Brazil Law, and the Combined Law. The other type of twinning is Inclined axis Twins which are defined by the Japan Law. Parallel Axis Twinning is the ultimate result of the formation of enantiomers. In chemistry enantiomers are represented by two molecules that are non-superimposable mirror images. An even a more simplified version would be the image of a person in a mirror and the actual person. They are not-superposable. Try this experiment: 1) make an "X" on one cheek, 2) have someone take a picture of you, 3) have them take a picture of your image in the mirror, 4) Note that you have two different pictures. You and your mirror image are Enantiomers. |
|
|
Right And Left Handed
Quartz Crystals
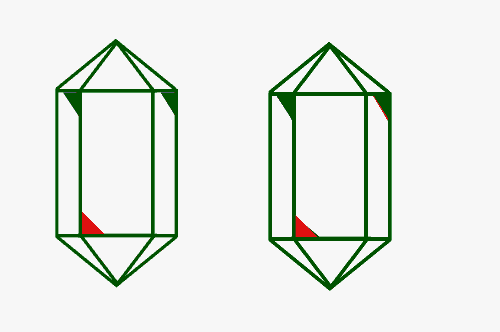
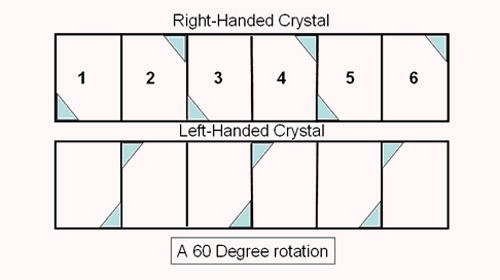
Enantiomers in quartz crystals leads to the classifications of Right and Left Handed Quartz crystals. The ideal quartz crystal would be doubly terminated with six-sided pyramids on each end of a six-sided prism. Remember that it very unusual to find a quartz crystal that is ideal. Usually you find only the prism with only one pyramid. A Right Handed quartz crystal will exhibit a beveled edge on the top right corner of every other prism face and a beveled edge on the lower left corner of the alternating prism faces. A Left Handed quartz crystal will exhibit a beveled edge on the top left corner of the prism face for every other prism face and a beveled edge on the right corner of the alternating prism faces.
|
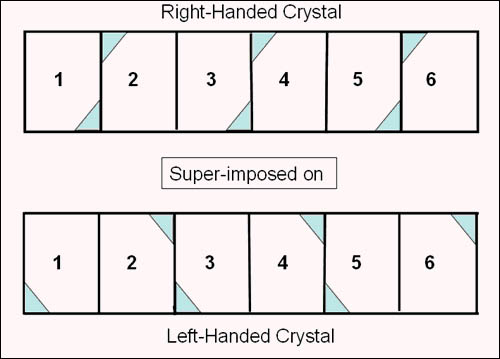
These two crystals are not superposable. They are enantiomers |
|
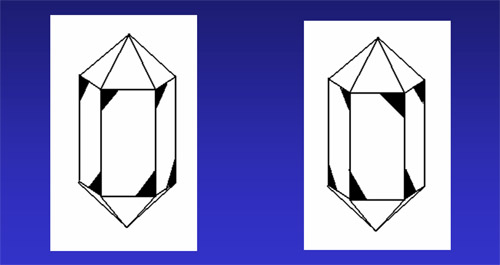
On the right are two identical doubly terminated right handed crystals . Rotation of one crystal will yield an identical crystal. This shows that the upper right hand corner of the crystal face is the same as the lower left corner of the alternate face when determining whether a crystal is right handed or left handed. |
Place your cursor over the picture above. The crystal on the right will rotate 180 degrees about an axis perpendicular to the plane of the monitor screen, Then it will rotate 60 degrees about an axis that is horizontal in the plane of the monitor. |
|
Look closely at the rotating crystal on the right. In the upper right corner of alternating prism faces there will appear a beveled edge. Usually you only find one or two faces that exhibit this beveled edge. It is unusual to find all three of the beveled edges on the alternating prism faces. It is even more difficult to find a doubly terminated quartz crystal that exhibits the beveled corners on the upper right corner of the three alternating prism faces and also exhibits the beveled corners on the lower left corner on the other three alternating prism faces. |
This is an example of a right handed crystal. |
|
Look closely at the rotating crystal on the right. In the upper left corner of alternating prism faces there will appear a beveled edge. Usually you only find one or two faces that exhibit this beveled edge. It is unusual to find all three of the beveled edges on the alternating prism faces. It is even more difficult to find a doubly terminated quartz crystal that exhibits the beveled corners on the upper left corner of the three alternating prism faces and also exhibits the beveled corners on the lower right corner on the other three alternating prism faces. |
This is an example of a left handed crystal. |
|
A More Technical Explanation
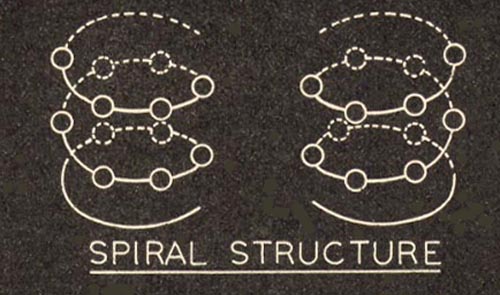
The right and left handedness of quartz is due to the arrangement of the SiO2 building blocks. As these building blocks are added to the structure they form spiral strings along the Z axis. These spirals are in either a clockwise or counter-clockwise rotation and cause quartz to rotate a plane of polarized light traveling parallel to the Z axis (often called the optical axis). If a crystal crystal rotates this plane of polarized light clockwise, it is classified as a right-handed quartz crystal. If it rotates the plane of polarized light in a counter-clockwise direction it is classified as a left-handed crystal. |
In this picture the + sign represents a silicon atom and the - sign represents an oxygen atom. This picture is from an article by Sidney X. Shore, "Inspection, Grading and Classification of Quartz" in "How Quartz Crystals are Manufactured",North American Phillips Co, Inc., 1944 |
In the Dauphine Law all six faces of the prism will exhibit either right or left handed characteristics. This means for a right handed crystal the top right corner of each prism face will have a beveled edge and the lower left hand corner of same prism face will have a beveled edge. All six prism faces would have the same arrangement of top right and lower left.
|
The Left and Right Handed DauphineTwin Law |
|
Most quartz crystals are attached to matrix. This results in a single termination where only half of the quartz crystal is observable. The crystal on the right is a left handed Dauphine twin that has a double termination. The prism face has a beveled edge on the top left corner and the lower right corner. When viewing a perfect crystal each of the six prism faces would show the same pattern.
|
Left Handed Dauphine Twin |
|
The Left Handed Dauphine Twin on the right shows the beveled edge on adjacent prism faces. Here each prism face would have a beveled edge ot the top left corner and the lower right corner. This crystal has a single termination where only half of the quartz crystal is observable.
|
This is a Left Handed Dauphine Twin. |
|
A Right Handed Dauphine Twin would have the opposite arrangement. Here each prism face would have a beveled edge ot the top right corner and the lower left corner. Remember a perfect Right or Left Handed Dauphine Twin crystal is very rare find. |
This is a Right Handed Dauphine Twin. |
|
A More Technical Explanation
A Dauphine Ttwin is often called an electrical twin. The right-handed and left-handed Dauphine Twins both result only in the orientation of the spiral strings of the SiO2 building blocks. Remember, a right-handed quartz crystal rotates a plane of polarized light clockwise. In a right-handed Dauphine Twin these spiral strings are rotated by 180 degrees. All the spirals are still clockwise. The same reasoning is true for left-handed Dauphine Twins except the spiral strings of SiO2 building blocks would be counter-clockwise. |
In this picture the + sign represents a silicon atom and the - sign represents an oxygen atom. This picture is from an article by Sidney X. Shore, "Inspection, Grading and Classification of Quartz" in "How Quartz Crystals are Manufactured",North American Phillips Co, Inc., 1944 |
|
In a Brazil Twin there will be a beveled edge on both the upper right hand corner and on the upper left hand corner of the same prism face. On the adjacent prism face there will be a beveled edge on both the lower right hand corner and the lower left hand corner. Ideally, this pattern would repeat itself around the prism faces.
|
|
|
Brazil Twins are often called optical twins. They are formed by the intergrowth of both a right-handed and a left-handed quartz crystal. Remember a right handed crystal |
This is a Brazil Twin. |
|
A More Technical Explanation A Brazil Twin is often called an optical twin. It is the result of the intergrowth of a right handed quartz crystal and a left handed quartz crystal where the clockwise and counter clockwise spiral strings are not rotated. Remember the right-handedness and left-handedness of quartz crystals is dependent on the clockwise or counter clockwise orientation of the spiraled strings of the SiO2 building blocks. A right-handed quartz crystal rotates a plane of polarized light clockwise and a left-handed quartz crystal rotates a plane of polarized light counter clockwise. Optically, these different rotations of polarized light can be observed parallel to the Z axis in a Brazil Twin.
|
In this picture the + sign represents a silicon atom and the - sign represents an oxygen atom.
This picture is from an article by Sidney X. Shore, "Inspection, Grading and Classification of Quartz" in "How Quartz Crystals are Manufactured",North American Phillips Co, Inc., 1944 |
 |
|
|
3) Combined Law In a Combined Twin Law there will be a beveled edge on both the upper right hand corner and on the lower right hand corner of the same prism face. On the adjacent prism face there will be a beveled edge on both the upper left hand corner and the lower left hand corner. Ideally, this pattern would repeat itself around the prism faces.
|
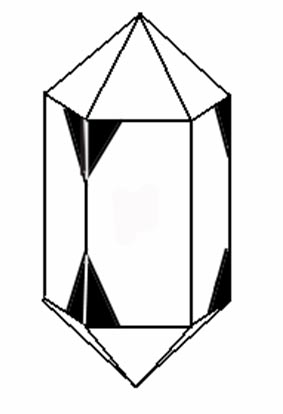 |
| Combined Twins are formed by the intergrowth of both a right-handed and a left-handed quartz crystal. Remember a right handed crystal will have a beveled edge on the top right hand side of one prism face and on the lower left hand side of the adjacent prism faces where as a left handed crystal will have a beveled edge on the top left hand side of one prism face and on the lower right hand side of the adjacent prism faces. If you rotate one crystal 180 around it's a axis, you will have a combined prism face similar to the one on the right. |
This is a Combined Twin. |
|
A More Technical Explanation A Combined Twin is the result of the intergrowth of a right-handed quartz crystal and a left-handed quartz crystal where the clockwise and counter clockwise spiral strings are rotated by 180 degrees. Remember the right-handedness and left-handedness of quartz crystals is dependent on the clockwise or counter clockwise orientation of the spiraled strings of the SiO2 building blocks. A right-handed quartz crystal rotates a plane of polarized light clockwise and a left-handed quartz crystal rotates a plane of polarized light counter clockwise. Optically, these different rotaotions of polarized light can be observed parallel to the Z axis in a Brazil Twin.
|
|
|
The Japan Law is characterized by the growth of two crystals at an inclined contact angle of 84o33' . This angle is slightly less than a right angle. There are numerous inclined axis twins, but the Japan law is classic. |
|
|
Carefully observe the crystal on the right as it rotates. It demonstrates the inclined axis law and at the same time one of the blades exhibits left-handedness. This crystal was purchased as a Japan twin, but it only exhibits the inclined axis twin law. It does not exhibit the Japan Law.
|
|
|
A More Technical Explanation The angle of the Japan Law twin law can be traced back to the plane that cuts the quartz crystal at the Miller Indices of 1,1,2,2
|
|